What is Creatine?
Creatine is a natural compound produced in the human body from the amino acids glycine and arginine, and using S-adenosyl-L-methionine (SAMe), a derivative of the amino acid methionine, as a cosubstrate. Creatine is used in a cellular system (the phosphocreatine system) that provides quick energy to help power tissues in moments of high energy output [1]. Because of this role, creatine is primarily found in tissues with high energy demands, particularly skeletal muscle, where around 95% of the body's creatine is stored, as well as the brain and cardiac muscle [2].
Although the human body can synthesize creatine [1,2], we get ~50% of our daily needs from animal foods in the diet, particularly from meat and fish. However, many people don’t get enough creatine from the diet and might not make sufficient creatine to optimize tissue status, especially vegans, vegetarians, and even omnivores who don’t eat much meat [3]. Since creatine is found almost exclusively in animal foods, persons following diets that limit or completely restrict animal foods may have lower body stores of creatine [4–7]. In fact, muscle creatine levels decreased significantly among women switching from an omnivorous to a vegetarian diet for 3 months, but were improved with the addition of 1 gram daily of creatine monohydrate [8]. Creatine supplements can help to maintain healthy tissue levels of creatine, particularly in physically active individuals.* But the benefits of creatine supplementation go beyond that, as we’ll see in this article.
Creatine Health Benefits
Creatine has a central role in supporting quick energy generation. Therefore, creatine is particularly important in tissues whose energy needs fluctuate and that have moments of high energy expenditure, like skeletal muscle and the brain. So naturally, these are also the tissues that benefit the most from creatine.*
Several studies have highlighted some of the potential benefits of creatine supplementation:
- Supports muscle strength* (PubMed: 32599716)
- Supports lean body mass* (PubMed: 40292479)
- Supports resistance training* (PubMed: 25993883)
- Supports memory, attention, and information processing speed* (PubMed: 39070254)
- Supports cognitive performance in healthy older individuals* (PubMed: 17828627)
How Does Creatine Work?
Adenosine triphosphate (ATP) is the energy molecule of cells. ATP is made up of an adenosine molecule bound to three phosphate groups. ATP is an energy-rich molecule because of the chemical bonds between its phosphate groups, which release a lot of energy when they are broken [9,10]. When ATP (the triphosphate version) is broken down to ADP (the diphosphate version), that energy is used to power cellular processes that require energy input, such as macromolecule synthesis, enzyme activity, nerve impulse conduction, and muscle contraction.
Most ATP is produced through a process called oxidative phosphorylation in mitochondria. However, in moments when energy expenditure is very high (in a brief, high-intensity exercise such as sprinting or weight training, as examples), ATP can be depleted, as this process is not fast enough to keep up with outstanding energy demands. But cells have a backup reserve of energy that can power a few extra seconds of peak energy expenditure—and that’s where creatine comes in.
Creatine is part of the phosphocreatine (or phosphagen) system for quick ATP regeneration. Phosphocreatine, also known as creatine phosphate, is a phosphorylated form of creatine, i.e., it is creatine with a high-energy phosphate group attached.
Phosphocreatine is used as a storage of phosphate. When ATP is being used at a high rate, an enzyme called creatine kinase quickly transfers a phosphate group from phosphocreatine to ADP to regenerate ATP, extending the cell’s energy capacity. When energy needs drop, phosphocreatine stores are restocked using ATP.
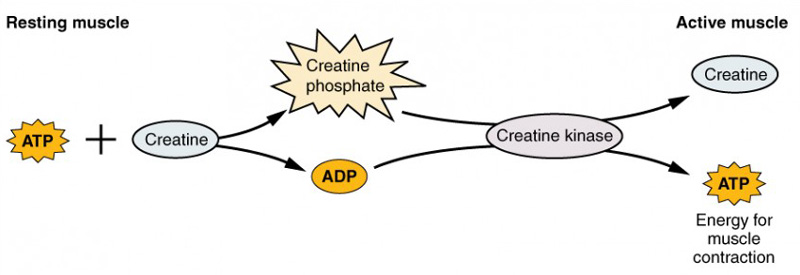
Figure 1. The phosphocreatine system. Source: OpenStax Anatomy and Physiology 2e, Chapter 10.3 - Muscle Fiber Contraction and Relaxation. CC BY 4.0
Energy-demanding tissues have a pool of phosphocreatine in their cells that can be mobilized in circumstances of very high energy expenditure, when the rate of ATP use exceeds the cells’ capacity to generate it by other metabolic pathways. This occurs, for example, during intense muscle contraction or neuronal activity. However, the phosphocreatine system has limited capacity. In skeletal muscle, phosphocreatine stores deplete quickly when the intensity is very high, usually in just 5 to 10 seconds of peak muscle power output. Therefore, the phosphocreatine energy system provides energy primarily to fuel only short-term high-intensity exercise.
How Does Creatine Support Exercise Performance?
Creatine supplementation supports muscle creatine and phosphocreatine pools, particularly when it’s accompanied by exercise [11–15]. This means that creatine supplementation may potentially support muscle capacity in moments of high power output. And indeed, studies indicate that creatine supports muscle strength and exercise performance during short-duration, high-intensity exercise, such as resistance training [15–21]. For endurance performance, on the other hand, creatine does not seem to be as beneficial, likely because muscles rely less on the phosphocreatine system for this type of exercise [1,21].*
Creatine also supports gains in muscle mass from exercise [1,15,16,21]. In one study, twelve weeks of resistance training promoted the growth of muscle fiber diameter by 6–15% in the control group; when coupled with creatine supplementation, resistance training promoted the growth of muscle fiber diameter by 35% [16].*
How Does Creatine Support Brain Function?
The brain synthesizes creatine, but it can also get creatine from the diet, as there are creatine transporters at the blood–brain barrier, in neurons, and in glial cells to take up creatine circulating in the blood [22,23]. Creatine supplementation has been shown to support brain creatine levels, although not to the same extent as it supports muscle levels [24–26].*
Similarly to skeletal muscles, the phosphocreatine system supports neuronal activity in moments of high energy demand [25,26]. The brain requires a lot of energy even for its basal functions, so during cognitively demanding tasks, when energy needs may peak, the support of the phosphocreatine system can be crucial for cognitive processing. Accordingly, studies have indicated that creatine supports brain function and cognitive performance in healthy individuals, particularly memory, reasoning, attention, and information processing speed [26–28]. Additionally, preclinical and human studies have indicated that creatine may also support neuroprotective functions [26,29–33].*
Creatine and Healthy Aging
By supporting muscle mass and function, creatine may indirectly support many other aspects of human biology that are essential for healthy aging.* That’s because skeletal muscle is also an important metabolic and endocrine organ: it is a major energy consumer, it influences our basal metabolic rate, it is the main tissue for the regulation of glucose and fat metabolism, it is the body’s largest storage reservoir of protein, it regulates insulin sensitivity, and it releases signaling molecules that influence other systems (e.g., nervous, endocrine, immune). This means that muscle has a pivotal role in metabolic function, whole-body homeostasis, and overall health [34,35].
Maintaining healthy muscles is important at any point in life, but even more so as we age and naturally begin to lose muscle mass [36]. Age-related muscle loss, which is called sarcopenia, occurs even in the context of healthy aging, but the more we can minimize or delay it, the healthier we may age. Therefore, by supporting muscle mass gains from exercise, creatine may also support healthy aging.*
Creatine has also been shown to support several aspects of healthy mitochondrial structure and function [29,37,38], which is a key aspect of healthy biological aging at the cellular level.* Healthy mitochondria are essential not only for an efficient generation of cell energy but also for the maintenance of healthy levels of reactive oxygen species (ROS) in cells. ROS are important signaling molecules, but they can be highly detrimental to cells when they accumulate, leading to oxidative stress and cellular aging. The major site of ROS production is the mitochondrial electron transport chain (ETC), where ATP is produced [39].
Mitochondrial function declines as we age—this is one of the twelve hallmarks of aging. This compromises the efficiency of the ETC and unbalances the production of ROS, which can lead to a progressive deterioration of cell and tissue function and, consequently, to an acceleration of the aging process [40,41]. Therefore, by supporting healthy mitochondrial function, creatine may also support healthy aging.*
What’s the Recommended Dose of Creatine for Health Benefits?
The efficacy of creatine supplementation is dose-dependent in the range it’s commonly used (up to about 5 grams a day). The recommended dose of creatine can vary depending on the purpose of supplementation. If the goal is to quickly help promote muscle creatine and phosphocreatine stores for exercise performance, higher servings (3-5 grams) are typically recommended [11,42,43].*
If the goal is to complement the dietary intake, a lower serving taken consistently over time may be sufficient. It’s been estimated that an omnivore diet provides about 1 gram of creatine a day [4,44] and that young adults make about 1 gram a day [4,6]. This combination of what we get in the diet and produce in the body is needed to compensate for the approximately 2 grams of creatine we lose every day. A dose of 1–3 grams of creatine can help offset our daily loss.*
Is Creatine Supplementation Safe?
Creatine is naturally found in the human body and is a component of a healthy diet. Creatine is also one of the most well-researched supplements with a strong safety profile. Studies have shown that creatine supplementation does not cause significant side effects at doses of up to 10 g/day administered long-term [45,46].*
There are anecdotal reports of gastrointestinal (GI) distress, bloating, and water retention, but research has found little evidence that creatine causes these side effects [49,50]. But it’s possible these adverse effects may occur when too much creatine is consumed at once [45,47], so reducing the amount of creatine being supplemented is a common strategy for managing GI and bloating issues.*
Creatine in Qualia Products
Creatine monohydrate has been the most scientifically studied form of creatine [28].* We included creatine monohydrate in Qualia Mitochondria+ to support mitochondrial function and muscle health, which are two key aspects of healthy aging and two core goals of our product.*
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
References
[1]J.T. Brosnan, M.E. Brosnan, Annu. Rev. Nutr. 27 (2007) 241–261.
[2]D.A. Bonilla, R.B. Kreider, J.R. Stout, D.A. Forero, C.M. Kerksick, M.D. Roberts, E.S. Rawson, Nutrients 13 (2021) 1238.
[3]D. Rogerson, J. Int. Soc. Sports Nutr. 14 (2017) 36.
[4]J.T. Brosnan, R.P. da Silva, M.E. Brosnan, Amino Acids 40 (2011) 1325–1331.
[5]M.E. Brosnan, J.T. Brosnan, Amino Acids 48 (2016) 1785–1791.
[6]R. Cooper, F. Naclerio, J. Allgrove, A. Jimenez, J. Int. Soc. Sports Nutr. 9 (2012) 33.
[7]M. Kaviani, K. Shaw, P.D. Chilibeck, Int. J. Environ. Res. Public Health 17 (2020) 3041.
[8]L. Blancquaert, A. Baguet, T. Bex, A. Volkaert, I. Everaert, J. Delanghe, M. Petrovic, C. Vervaet, S. De Henauw, D. Constantin-Teodosiu, P. Greenhaff, W. Derave, Br. J. Nutr. 119 (2018) 759–770.
[9]J.M. Berg, J.L. Tymoczko, G.J. Gatto, L. Stryer, eds., Biochemistry, 8th ed, W.H. Freeman and Company, 2015.}[10]D.L. Nelson, M.M. Cox, Lehninger Principles of Biochemistry, 7th Edition, W. H. Freeman and Company, 2017.
[11]R.C. Harris, K. Söderlund, E. Hultman, Clin. Sci. (Lond.) 83 (1992) 367–374.
[12]A. Casey, D. Constantin-Teodosiu, S. Howell, E. Hultman, P.L. Greenhaff, Am. J. Physiol. 271 (1996) E31–7.
[13]P.L. Greenhaff, K. Bodin, K. Soderlund, E. Hultman, Am. J. Physiol. 266 (1994) E725–30.
[14]J.J. Brault, T.F. Towse, J.M. Slade, R.A. Meyer, Int. J. Sport Nutr. Exerc. Metab. 17 (2007) 624–634.
[15]D.G. Burke, P.D. Chilibeck, G. Parise, D.G. Candow, D. Mahoney, M. Tarnopolsky, Med. Sci. Sports Exerc. 35 (2003) 1946–1955.
[16]J.S. Volek, N.D. Duncan, S.A. Mazzetti, R.S. Staron, M. Putukian, A.L. Gómez, D.R. Pearson, W.J. Fink, W.J. Kraemer, Med. Sci. Sports Exerc. 31 (1999) 1147–1156.
[17]S.L. Nissen, R.L. Sharp, J. Appl. Physiol. 94 (2003) 651–659.
[18]R.B. Kreider, Mol. Cell. Biochem. 244 (2003) 89–94.
[19]L.A. Gotshalk, W.J. Kraemer, M.A.G. Mendonca, J.L. Vingren, A.M. Kenny, B.A. Spiering, D.L. Hatfield, M.S. Fragala, J.S. Volek, Eur. J. Appl. Physiol. 102 (2008) 223–231.
[20]L.A. Gotshalk, J.S. Volek, R.S. Staron, C.R. Denegar, F.C. Hagerman, W.J. Kraemer, Med. Sci. Sports Exerc. 34 (2002) 537–543.
[21]J.D. Branch, Int. J. Sport Nutr. Exerc. Metab. 13 (2003) 198–226.
[22]S. Ohtsuki, M. Tachikawa, H. Takanaga, H. Shimizu, M. Watanabe, K.-I. Hosoya, T. Terasaki, J. Cereb. Blood Flow Metab. 22 (2002) 1327–1335.
[23]O. Braissant, C. Bachmann, H. Henry, Subcell. Biochem. 46 (2007) 67–81.
[24]P. Dechent, P.J.W. Pouwels, B. Wilken, F. Hanefeld, J. Frahm, Am. J. Physiol. Regul. Integr. Comp. Physiol. 277 (1999) R698–R704.
[25]E. Dolan, B. Gualano, E.S. Rawson, EJSS (Champaign) 19 (2019) 1–14.
[26]H. Roschel, B. Gualano, S.M. Ostojic, E.S. Rawson, Nutrients 13 (2021) 586.
[27]K.I. Avgerinos, N. Spyrou, K.I. Bougioukas, D. Kapogiannis, Exp. Gerontol. 108 (2018) 166–173.
[28]C. Xu, S. Bi, W. Zhang, L. Luo, Front. Nutr. 11 (2024) 1424972.
[29]P. Klivenyi, R.J. Ferrante, R.T. Matthews, M.B. Bogdanov, A.M. Klein, O.A. Andreassen, G. Mueller, M. Wermer, R. Kaddurah-Daouk, M.F. Beal, Nat. Med. 5 (1999) 347–350.
[30]G.J. Brewer, T.W. Wallimann, J. Neurochem. 74 (2000) 1968–1978.
[31]B. Valastro, A. Dekundy, W. Danysz, G. Quack, Behav. Brain Res. 197 (2009) 90–96.
[32]R.T. Matthews, L. Yang, B.G. Jenkins, R.J. Ferrante, B.R. Rosen, R. Kaddurah-Daouk, M.F. Beal, J. Neurosci. 18 (1998) 156–163.
[33]P.G. Sullivan, J.D. Geiger, M.P. Mattson, S.W. Scheff, Ann. Neurol. 48 (2000) 723–729.
[34]H.J. Coelho-Junior, A. Picca, R. Calvani, M.C. Uchida, E. Marzetti, Exp. Gerontol. 127 (2019) 110715.
[35]K.K. Baskin, B.R. Winders, E.N. Olson, Cell Metab. 21 (2015) 237–248.
[36]M.R. Deschenes, Sports Med. 34 (2004) 809–824.
[37]E. Barbieri, M. Guescini, C. Calcabrini, L. Vallorani, A.R. Diaz, C. Fimognari, B. Canonico, F. Luchetti, S. Papa, M. Battistelli, E. Falcieri, V. Romanello, M. Sandri, V. Stocchi, C. Ciacci, P. Sestili, Oxid. Med. Cell. Longev. 2016 (2016) 5152029.
[38]L.M. Rambo, L.R. Ribeiro, I.D. Della-Pace, D.N. Stamm, R. da Rosa Gerbatin, M. Prigol, S. Pinton, C.W. Nogueira, A.F. Furian, M.S. Oliveira, M.R. Fighera, L.F.F. Royes, Amino Acids 44 (2013) 857–868.
[39]M. Schieber, N.S. Chandel, Curr. Biol. 24 (2014) R453–62.
[40]J.A. Amorim, G. Coppotelli, A.P. Rolo, C.M. Palmeira, J.M. Ross, D.A. Sinclair, Nat. Rev. Endocrinol. 18 (2022) 243–258.
[41]C. López-Otín, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer, Cell 186 (2023) 243–278.
[42]E. Hultman, K. Söderlund, J.A. Timmons, G. Cederblad, P.L. Greenhaff, J. Appl. Physiol. 81 (1996) 232–237.
[43]P.D. Balsom, K. Söderlund, B. Ekblom, Sports Med. 18 (1994) 268–280.
[44]M.E. Brosnan, J.T. Brosnan, Amino Acids 48 (2016) 1785–1791.
[45]G.J. Groeneveld, C. Beijer, J.H. Veldink, S. Kalmijn, J.H.J. Wokke, L.H. van den Berg, Int. J. Sports Med. 26 (2005) 307–313.
[46]A. Bender, W. Samtleben, M. Elstner, T. Klopstock, Nutr. Res. 28 (2008) 172–178.
[47]A. Shao, J.N. Hathcock, Regul. Toxicol. Pharmacol. 45 (2006) 242–251.








No Comments Yet
Sign in or Register to Comment