We teamed up with Reputable Health (www.reputable.health) to understand how real users responded to Qualia Night, our sleep support formula. Here's what was done—and what we found.
HOW THE STUDY WORKED
The purpose of this study was to better understand participant-reported sleep and stress responses to Qualia Night using validated surveys, and to explore Reputable Health's ability to integrate wearable device data.
Reputable Health conducted a real-world evidence pilot study with 35 healthy adults enrolled in a 28-day program. Participants took Qualia Night at dinner or in the early evening following a 5 days on and 2 days off protocol over the course of the study.
Participants completed two validated surveys—the Pittsburgh Sleep Quality Index (PSQI) and the Depression, Anxiety & Stress Scales (DASS)—before and after taking Qualia Night. Continuous biometric tracking was done using ŌURA Ring as the wearable device.
Twenty-eight participants successfully completed the full protocol, representing an 82% completion rate. Twenty-one (75%) of the successful completers were females and 7 (25%) were males.
RESULT HIGHLIGHTS
Qualia Night Improved Sleep Quality and Next-Day Function†*
- Sleep Quality: The Sleep Quality subscale of the PSQI assesses how satisfied a person is with their sleep and how well they perceive it to be. Sleep Quality scores improved significantly compared to baseline by 25% (p = 0.021).†*
- Daytime Dysfunction: The Daytime Dysfunction subscale of the PSQI assesses how poor sleep affects a person’s ability to function effectively during the day. Daytime Dysfunction scores improved significantly compared to baseline by 35% (p = 0.007).†*
- Sleep Latency: The Sleep Latency subscale of the PSQI assesses how quickly a person is able to fall asleep. Sleep Latency scores improved by 13% compared to baseline; however this was not statistically significant (p = 0.141).†*
- Global Score: The Global score is the overall score after combining all subscales from the PSQI. It showed a 14% improvement that trended towards statistical significance (p = 0.083).†*
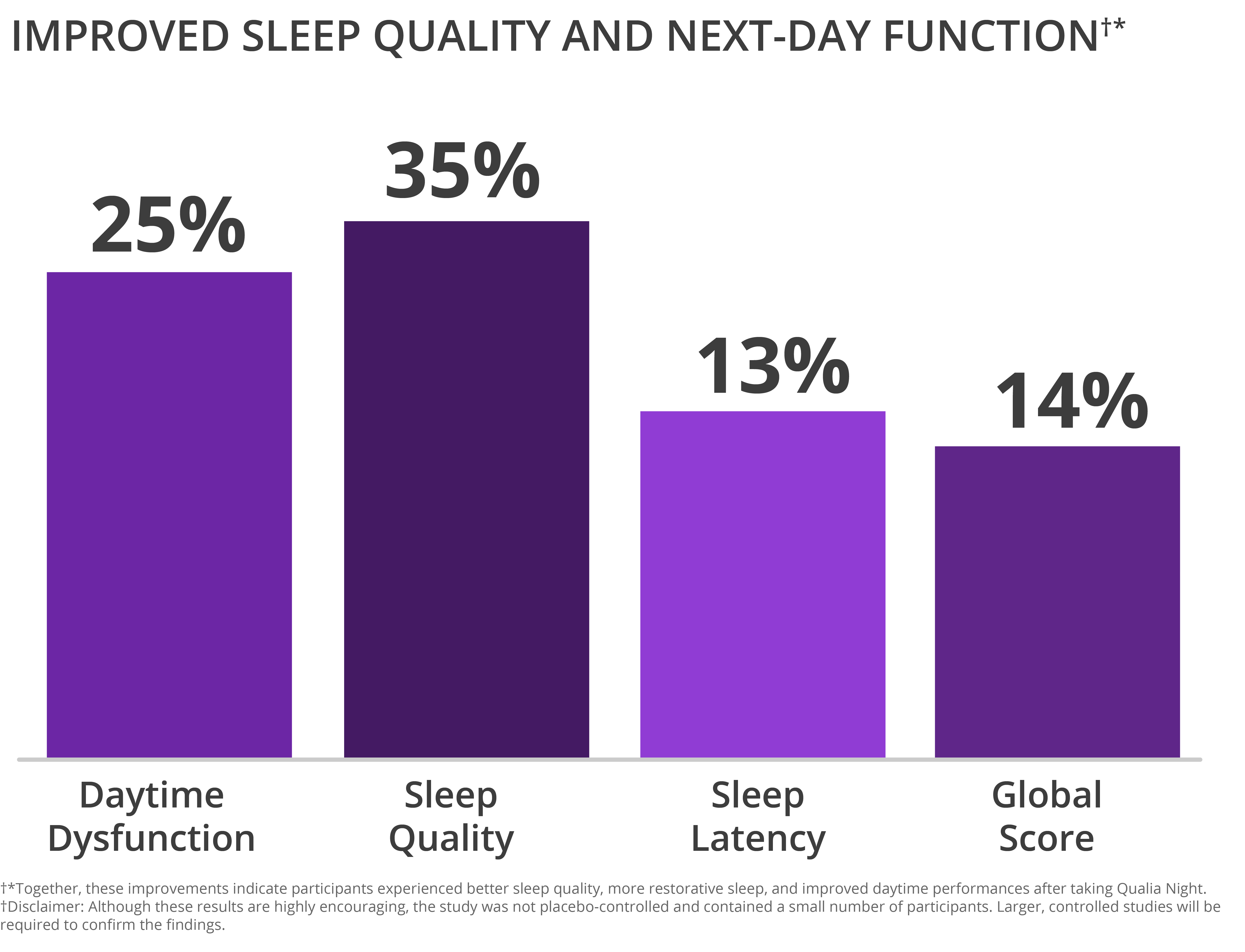
Together, these improvements indicate participants experienced better sleep quality, more restorative sleep, and improved daytime performances after taking Qualia Night.†*
Reduced Feelings of Stress, Anxiety, and Low Mood†*
- Anxiety-Stress Scores: The Anxiety-Stress subscale scores of the DASS provides an indication of how anxious and stressed a person is feeling. This score decreased significantly compared to baseline by 39% (p < 0.001).†*
- Depression Scores: The Depression subscale scores of the DASS provides an indication of how low mood may be impacting a person. This score decreased significantly compared to baseline by 27% (p = 0.035).†*
- Overall DASS Composite: The Overall DASS score is the combination of the above scores and provides an indication of a person’s level of distress. It decreased significantly compared to baseline by 34% (p = 0.003).†*
Participants reported feeling notably calmer, less stressed and more emotionally balanced after four weeks taking Qualia Night.†*
Positive Trends in Objective Biometrics†*
Though not statistically significant in this initial pilot, wearable data indicated a few promising trends†*:
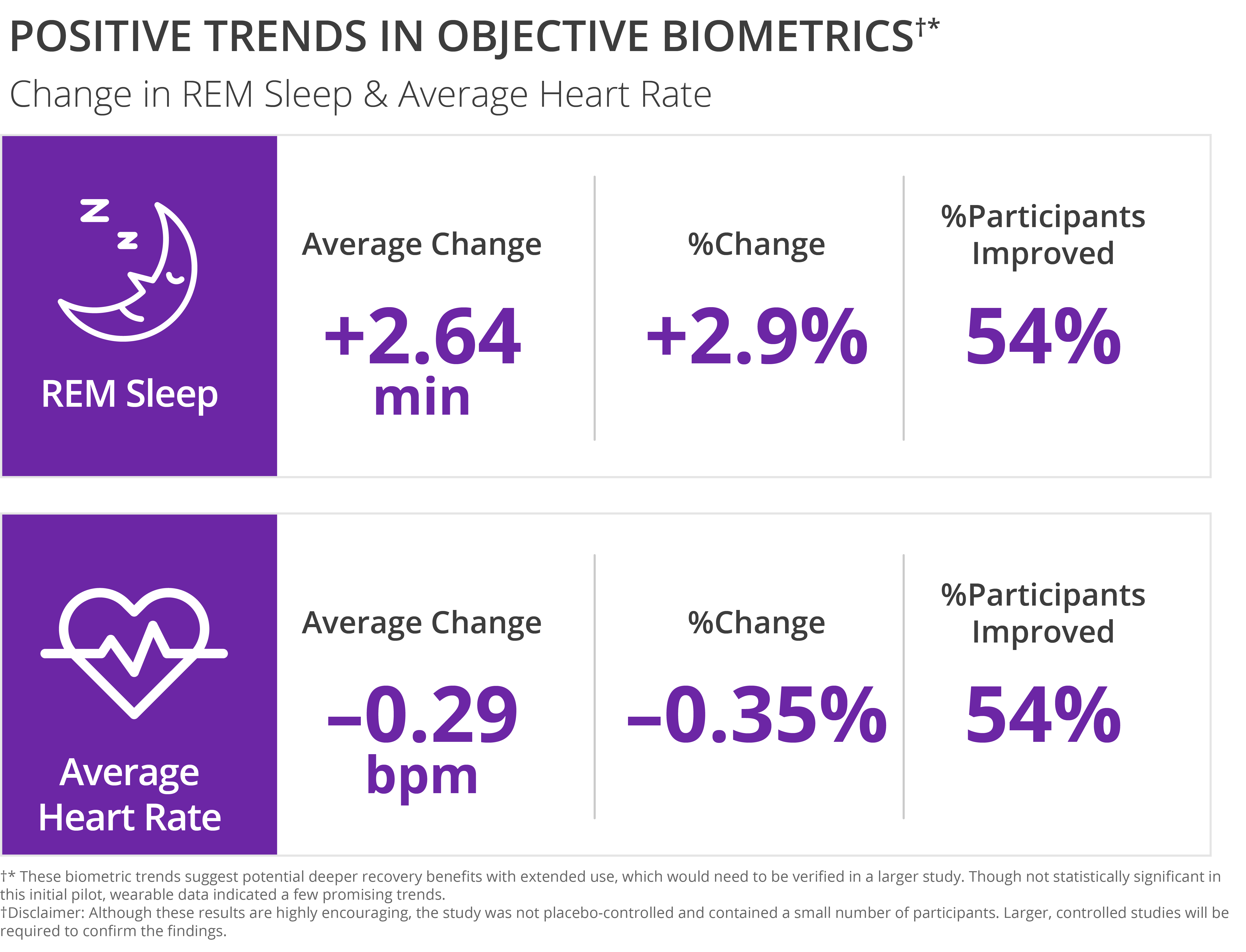
These biometric trends suggest potential deeper recovery benefits with extended use, which would need to be verified in a larger study.†*
WHAT THIS MEANS FOR YOU
- More Restorative Sleep: Users experienced improved sleep quality and reduced daytime grogginess within just four weeks.†*
- Calmer Days: Significant decreases in stress, anxiety, and low mood help create smoother transitions from day to night.†*
- Early Physiological Benefits: Encouraging shifts in REM sleep and heart-rate metrics indicate possible deeper health benefits, deserving of further exploration.†*
LOOKING AHEAD
Future research with placebo controls, larger participant groups, and longer durations will further confirm and expand these promising results.†* This study’s results focused on the DASS score as an indicator of emotional well-being. This study did not diagnose or study any disorder or disease.
†Disclaimer: Although these results are highly encouraging, the study was not placebo-controlled and contained a small number of participants. Larger, controlled studies will be required to confirm the findings.
*These statements have not been evaluated by the Food and Drug Administration. The products and information on this website are not intended to diagnose, treat, cure or prevent any disease. The information on this site is for educational purposes only and should not be considered medical advice. Please speak with an appropriate healthcare professional when evaluating any wellness related therapy. Please read the full medical disclaimer before taking any of the products offered on this site.


No Comments Yet
Sign in or Register to Comment