As part of our product development process, Qualia Stem Cell was put to the test to find out how users would respond. Here’s what we did, and what we discovered.
HOW THE QUALIA STEM CELL PILOT STUDY WORKED
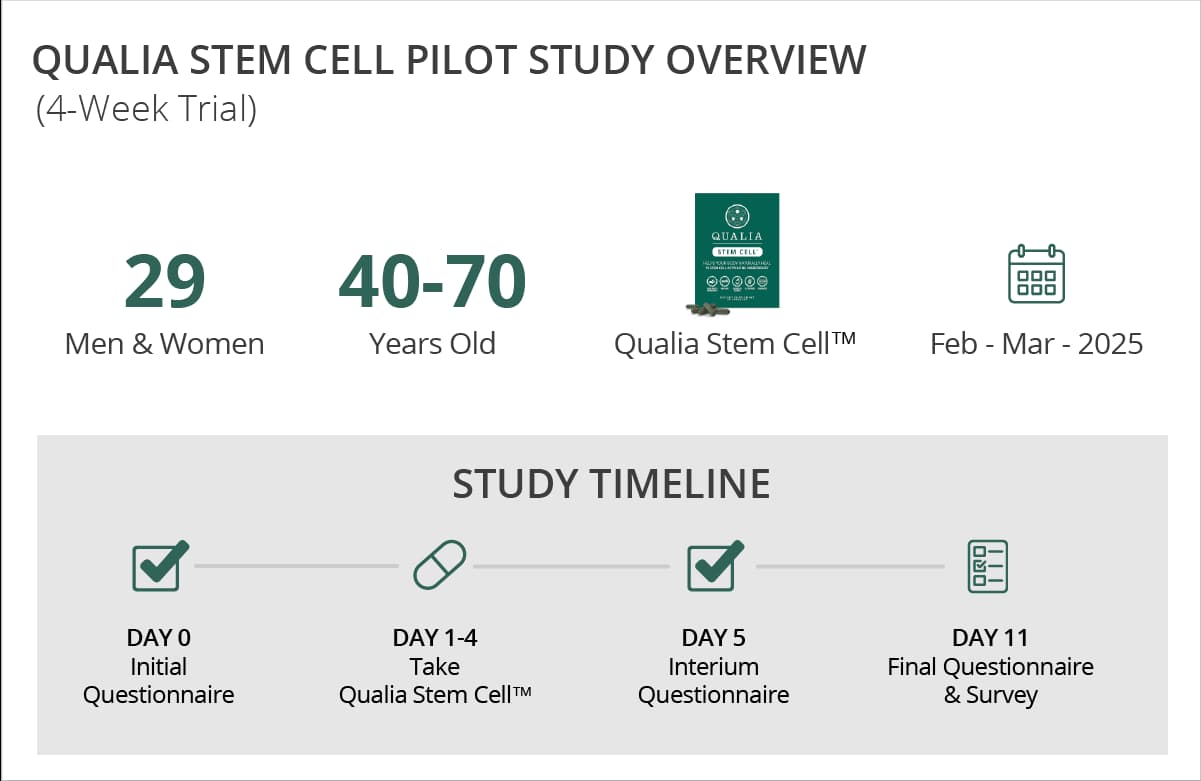
Thirty volunteers aged 40-70 agreed to be a part of our beta study. We tested two formulas that overlapped in core ingredients—a 6 capsule and 8 capsule formula—to see if people responded more favorably to one over the other. The study was 4 days in length with participants randomly allocated to 2 groups: (1) the 6 capsule group, or (2) the 8 capsule group. Twenty-nine of these participants completed the 4 day study (a high percentage of completion), likely due to the short duration and tolerability of both formulas.
Qualia Stem Cell was formulated to support the health and performance of the body’s stem cells over time. It should be thought of as something that provides more support for healing with repeated cycles of use. Considering the brief duration of this study and the product's design not being intended for short-term "feeling" or "experiencing,” our primary outcomes were to assess safety & tolerability by asking about the common unwanted effects associated with taking any new compound, including placebos. Volunteers were asked to rate their experiences of areas such as occasional mood complaints, gastrointestinal disturbances, body discomfort, etc. at baseline and during the study. *
While we did not anticipate any substantial felt experiences after a single 4-day dosing cycle—healing and rejuvenation benefits of enhanced stem cell function may not be apparent after only 4 days—we were curious to explore whether any aging symptoms or healing experiences were reported by participants. Secondary exploratory outcomes included (1) symptoms of aging and (2) more general questions about healing and well-being. For (1), these questionnaires asked about common health challenges that occur with aging for psychological (irritability, mood, nervousness, etc.), somatic (general well-being, sleep, feelings of exhaustion, etc), and sexual health. For (2), these survey questions asked about tissue healing or rejuvenation, energy levels, focus and clarity, improvements in aches or discomfort, changes in skin or nails, and subjective well-being. *
On days 0 (prior to supplementation) and 5 (after supplementation) of the study, participants completed baseline safety & tolerability questionnaires and reported their observations from secondary exploratory outcomes. From days 1 - 4, participants consumed Qualia Stem Cell and reported daily check-ins and safety & tolerability outcomes. A follow up survey and another exploration of secondary outcomes were conducted 5 - 7 days after the final dose of Qualia Stem Cell.
RESULT HIGHLIGHTS
Qualia Stem Cell was well-tolerated†*
For our primary outcomes of safety and tolerability, no significant differences between the 6 and 8 capsule groups were observed (p > 0.05)—meaning both formulations were well tolerated compared to one another. Both versions were also well-tolerated compared to volunteers’ baselines ratings.
Qualia Stem Cell supports healthy aging and well-being†*
For our secondary exploratory outcomes, twenty-nine participants completed these questionnaires: 14 males and 15 females. The symptoms of aging scores decreased from baseline in males by ~30% with no difference compared to the 6 and 8 capsule group, while the scores decreased in females more in the 6 capsule group (~42%) compared to the 8 capsule group (~14%). For the survey questions about healing and well-being, overall, 19% reported tissue healing or rejuvenation, 33% noticed energy changes or improved well-being, 27% noticed improvements in joint/muscle function, 30% noticed improvements in focus/mental clarity, and 7% noticed improvements in skin or nails. Given the short duration of the study, it was encouraging to find that 41% of participants noticed benefits in 1 or more of these areas related to healing and well-being.†*
Qualia Stem Cell was rated favorably by most users††*
Five to seven days after completing the study, participants who had taken Qualia Stem Cell for 4 days were asked to describe their overall experience. Twenty-seven participants completed this survey. Answer choices were “Excellent,” “Good,” “No Effect,” “Poor,” and “Very Poor.” Fifty-six percent of participants who took Qualia Stem Cell described their experience as excellent or good. No participants reported “Poor” or “Very Poor” experiences, further supporting the tolerability of Qualia Stem Cell. Overall, 89% of participants reported an interest in purchasing the product, with 44.4% answering “yes” and another 44.4% “maybe” about their purchasing interest. The average rating of this product on a scale of 1-5 stars was 3.89.††*
†Disclaimer: Although these results are highly encouraging, these findings are not statistically significant and the study was not placebo-controlled. Larger, controlled studies will be required to confirm the findings.
††Disclaimer: The survey contained a small number of participants who received the product for free.
*These statements have not been evaluated by the Food and Drug Administration. The products and information on this website are not intended to diagnose, treat, cure or prevent any disease. The information on this site is for educational purposes only and should not be considered medical advice. Please speak with an appropriate healthcare professional when evaluating any wellness related therapy. Please read the full medical disclaimer before taking any of the products offered on this site.


No Comments Yet
Sign in or Register to Comment